One in 77 women will develop ovarian cancer in her lifetime. Because ovarian cancer has no defined symptoms, most women will be diagnosed at a late stage of the disease where metastatic lesions are found dispersed throughout the abdomen. Ovarian cancer is currently the fifth most common cause of cancer-related deaths in women. With new technology being developed at the Harper Cancer Research Institute, the ovarian cancer surgery success rate may ultimately improve significantly.
Ovarian cancer distinctly spreads in the abdomen generating many sites of cancer along the peritoneum, the tissue lining the abdominal cavity. The most common treatment of ovarian cancer includes a debulking surgery, or the removal of cancerous lesions from the abdominal/peritoneal cavity. Currently, ovarian cancer surgeries are considered a ‘success’ with the removal of all tumors 1cm or larger. However, with research being developed at the Harper Cancer Research Institute, this goal could soon become the removal of tumors as small as 1mm.
The major challenge lies in distinguishing the healthy cells from the cancerous cells. Anatomy text books are often color-coded and thus the identification of the body parts is simplified. However, in reality the tissue structure within the human body actually appears as a blend of pinks varying to reddish in color and is very difficult to decipher. There may be little to no visible distinction between healthy tissue and cancer cells or even nerves.
Enter a multi-colored, fluorescent signaling, targeted molecule tagging system. Bradley Smith, a principal Investigator at the Harper Cancer Research Institute, Director of the Notre Dame Integrated Imaging Facility (NDIIF), and Emil T. Hofman Professor of Chemistry at the University of Notre Dame, has developed targeted, or “smart,” molecules that seek out and bind to specified cells. These molecules attach to the cancer cells and emit a fluorescent signal when viewed through a fluorescent camera and glow in a specified color.
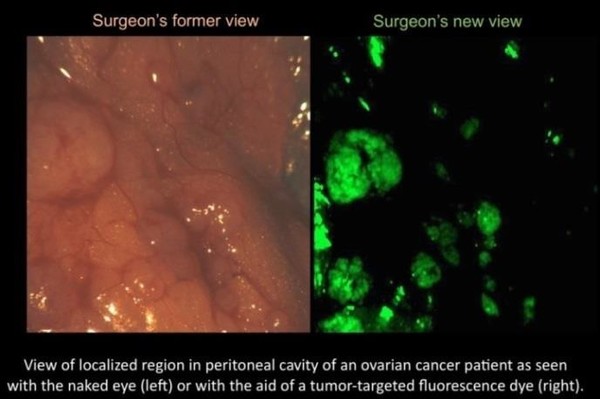
Currently, only a few non-targeted or “dumb” molecules have been approved by the Federal Drug Administration (FDA). While these molecules do work in the human liver and brain, the real need is for targeted or “smart” molecules. These non-toxic fluorescent molecules are the next generation of fluoresce guided surgery. A likely application of Smith’s research would be for the surgeon to dose patients with a targeted molecule before surgery, and then overlay the fluorescent imagery to create an illuminated map of the cancer cell locations. At the same time, other smart molecules would attach to key anatomical structures like nerves and appear in a different designated color – to alert the surgeon so they would not be inadvertently cut. The surgeon would then be able to locate and remove all tumors greater than 1mm.
“When a surgeon extracts a tumor, the immediate area around it, referred to as the ‘margin,’ is also removed. After surgery, the margins are tested for the presence of cancerous cells –a negative result indicates that there are no cancerous cells in the margins. A positive result was found in 9% of all the surgeries studied over a 14 year period. Unfortunately, however, 30% of ovarian cancer surgeries during that period had positive margins – a number I find to be most unacceptable” said Smith.
Smith’s expertise in chemistry is helping to develop targeted fluorescent molecules that are non-toxic and capable of deep, high resolution tissue presentation. “They resemble tiny spies that seek out the cancerous tissue and then their illumination reports the information to the camera.” Smith is currently collaborating with a local surgeon on this exciting development.
“Being a member of the Harper Cancer Research Institute has greatly helped make the connections necessary to further this project. As a chemist, I have only a moderate understanding of cancer as a clinical disease. Being able to talk with people with clinical information, such as Sharon Stack [Ann F. Dunne & Elizabeth Riley Director, Harper Cancer Research Institute and Kleiderer-Pezold Professor of Biochemistry at the University of Notre Dame], allows me to see the clinical relevance to my research. Likewise, working with electrical engineers like Scott Howard, Assistant Professor of Electrical Engineering at the University of Notre Dame and an imaging specialist, has been greatly helpful to my research.”
Smith’s research has recently led to a $1.5 million 5 year National Institutes of Health (NIH) grant. His research is a prime example of the multidisciplinary focus that is the cornerstone of HCRI. Befittingly, his office is located in McCourtney Hall – a building dedicated to research in the molecular sciences and engineering at the University of Notre Dame. Researchers from two Colleges – Engineering and Science–join forces to tackle three key programmatic areas: analytical sciences and engineering, chemical and biomolecular engineering, and drug discovery.
The Harper Cancer Research Institute researchers are dedicated to conducting innovative and integrative research that confronts the complex challenges of cancer. From ovarian to pancreatic, breast to bone, the University of Notre Dame and the Indiana University School of Medicine–South Bend are working together to discover novel therapeutics and diagnostics to better fight the cancers that afflict millions of people each year.
Originally published by at harpercancer.nd.edu on November 15, 2016.
